Abstract
Background: LentiGlobin Drug Product (DP) contains autologous CD34+ cells transduced with the betibeglogene darolentivec (BB305) lentiviral vector, encoding a human β-globin gene with a point mutation (AT87Q) that confers anti-sickling properties similar to those with γ-globin. Human proof of concept for LentiGlobin treatment in transfusion-dependent β-thalassemia (TDT) and severe sickle cell disease (SCD) has been established in HGB-205 (NCT02151526), the original clinical study of gene therapy for hemoglobinopathies. Here, we provide an update on 5 previously-reported patients (4 TDT, 1 SCD) as well as early data from 2 additional treated patients with SCD.
Methods: HGB-205 enrolled patients (5−35 years old) with TDT (≥100 mL/kg of packed red blood cells [pRBCs] per year) or severe SCD (e.g., failed hydroxyurea treatment and ≥2 acute chest syndrome episodes [ACS] or ≥2 vaso-occlusive crises [VOC] in the preceding year/year prior to regular transfusions). Following mobilization/apheresis for TDT or bone marrow harvest for SCD, autologous CD34+ cells were transduced with the BB305 lentiviral vector. Patients underwent myeloablative conditioning with busulfan, were infused with transduced cells and monitored for hematologic engraftment, vector copy number (VCN), transgenic hemoglobin (HbAT87Q) levels, and adverse events (AEs). Transfusion requirements and biological markers for TDT, and VOCs for SCD were also assessed.
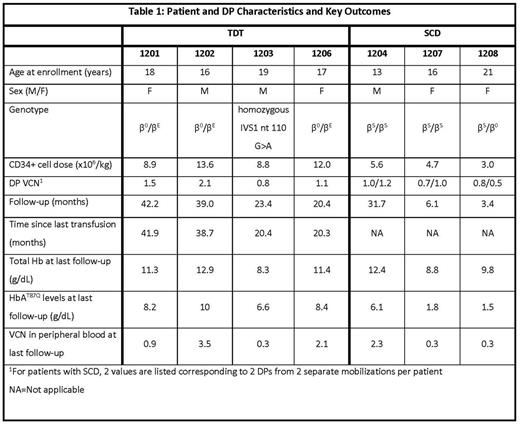
Results: As of June 2, 2017, 4 patients with TDT and 3 patients with severe SCD have received LentiGlobin DP; median follow-up is 23.4 (range, 3.4 - 42.2) months. Patient and DP characteristics and key outcomes for these 7 patients are shown in Table 1. All patients engrafted successfully. The toxicity profile was consistent with myeloablative conditioning with single-agent busulfan, with no DP-related ≥ grade 3 AEs or serious AEs and no evidence of clonal dominance reported to date. For patients with TDT, VCN, total Hb and HbAT87Q levels in peripheral blood remain stable, with no evidence of decreasing efficacy (Table 1). All patients with TDT remain free of RBC transfusions. In all 3 β0/βE patients, Hb levels are stable near or within the normal range. Importantly, iron burden and biological hallmarks of dyserythropoiesis have largely returned to normal. Two patients with TDT have discontinued iron chelation therapy and transitioned to therapeutic phlebotomy while the third patient (1202) has been able to discontinue both.
All 3 patients with SCD showed increase in HbAT87Q production through 6 months or last follow-up. The first treated patient with severe SCD (1204) had no clinical symptoms or complications of SCD until, at about 30 months post-treatment, the patient developed a VOC following an episode of acute gastroenteritis with fever and dehydration, and was subsequently hospitalized. His total Hb levels (12.4 g/dL), HbAT87Q (6.1 g/dL) and peripheral blood VCN levels (2.3) have remained stable at 30 months of follow-up. The 2 recently treated patients with SCD (1207 and 1208) have 6 and 3 months of follow-up, have not undergone transfusions since Day 21 and 15 post-infusion, and have total Hb of 8.8 g/dL and 9.8 g/dL and HbAT87Q of 1.8 and 1.5 g/dL, respectively as of last follow-up. At approximately 6 months post-treatment, patient 1207 experienced an episode of ACS and was hospitalized. Total % anti-sickling Hb ([HbAT87Q + HbF + HbA2]/Total Hb * 100) in the 3 patients with SCD was 52%, 39% and 36% as of last follow-up. Levels of unconjugated bilirubin, lactate dehydrogenase and reticulocyte count had improved compared to screening in the 2 patients with SCD (1204 and 1207) who had ≥ 6 months follow-up.
Summary: Data from this pioneering phase 1/2 clinical study continue to suggest that treatment with LentiGlobin DP elicits sustained HbAT87Q levels which may be sufficient to alleviate the clinical and biochemical effects of severe SCD and TDT, with an acceptable safety profile. In patients with TDT, sustained levels of HbAT87Q with longer term follow-up, abatement of dyserythropoeisis and discontinuation of iron chelation therapy confirm the results seen to date. For patients with SCD, the marked clinical improvement in 1 patient with nearly 2.5 years of follow-up and increasing HbAT87Q levels in recently treated patients suggest gene therapy may be a promising option. Follow-up data on the 3 patients with SCD and 2-year primary follow-up for patients with TDT will be presented.
Payen: bluebird bio: Honoraria, Research Funding. Brousse: Add Medica: Membership on an entity's Board of Directors or advisory committees. Bartolucci: Novartis: Consultancy; AddMedica: Consultancy, Research Funding. Beuzard: bluebird bio: Consultancy, Equity Ownership. Asmal: bluebird bio: Employment, Equity Ownership. Miller: bluebird bio: Employment, Equity Ownership. De Montalembert: Addmedica: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Leboulch: bluebird bio: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Ribeil: Addmedica: Membership on an entity's Board of Directors or advisory committees; Vitalaire: Other: Non-financial support; Novartis: Other: Non-financial support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal